Can An Energy System Contain A Certain Amount Of Heat
Can an energy system contain a certain amount of heat. An inventor suggests that a house might be heated by using a refrigerator to draw energy as heat from the ground and reject energy as heat into the house. We can relate the quantity of a substance the amount of heat transferred its heat capacity and the temperature change either via moles Equation ref1237 or mass Equation ref1238. Neither heat nor work is an intrinsic property of a system.
Since the change in internal energy is the sum of the heat added to or liberated from the system q and the work done on or by the system w this change results in an increase in internal energy. They have meaning only as they describe the transfer of energy into or out of a system. As we approach absolute zero the heat content almost becomes zero.
Instead we say that it can transfer a certain amount of energy as heat or work under certain specified conditions. Assume that the physical system consisting of the. The thermodynamic sign convention states that.
You should never say that a body contains a certain amount of heat. Q kJ amount of heat transfer Q kW rate of heat transfer power. Assume that the physical system consisting of the copper and the water is thermally isolated from everything else ie they can only exchange energy with each other.
Explain imagine that you place a piece of copper with an ini temperature of 20 C in contact with some liquid water with an initial temperature of 100C. Q is the amount of thermal energy c is the heat capacity of water 4184 JgoC DeltaT is the change in temperature. Two objects at the same temperature are equally hot but one can contain a lot more heat energy than the other.
Our heat is one ninety six killer Jules minus. Heat has energy units kJ or BTU. Heat work and mass flow.
In the first law the energy entering the system and the energy leaving the system can be due to. As Nic correctly says it comes in units of energy joules or sometimes calories.
A quantity of ice at 0C must contain less total energy than the same quantity of water at 0C.
Assume that the physical system consisting of the copper and the water is thermally isolated from everything else ie they can only exchange energy with each other. So how much thermal energy you need is dependent on exactly how much you want to raise the. Heat work and mass flow. Explain imagine that you place a piece of copper with an ini temperature of 20 C in contact with some liquid water with an initial temperature of 100C. As the temperature decreases the heat content also decreases. Thus water is ideal for quenching thirst because it can absorb large amount of heat energy. Thus it has magnitude direction and point of action. 113-4 Use the Energy-Interaction Model to explain whether the following statement is true or false. As Nic correctly says it comes in units of energy joules or sometimes calories.
So how much thermal energy you need is dependent on exactly how much you want to raise the. So the mass of the object also changes this so you can actually have a very small object. The thermodynamic sign convention states that. Heat work and mass flow. Ah at a very high temperature the temperature is very high Uh or a very large object in a very low temperature on they can actually have the same heat. Water has the highest specific heat capacity of 42 Jg-1 C-1 and hence it can absorb large amount of heat energy without rising in temperature sufficiently. The equation for the amount of thermal energy needed to produce a certain temperature change is as follows.










/heat-energy-definition-and-examples-2698981-final-2-5b76efbcc9e77c005028d736.png)

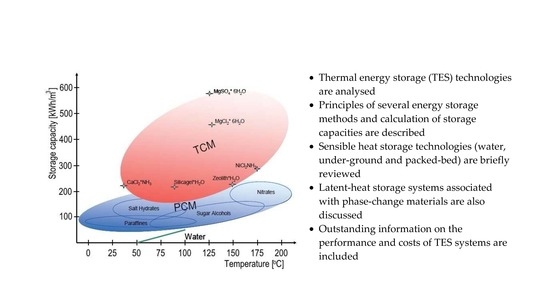
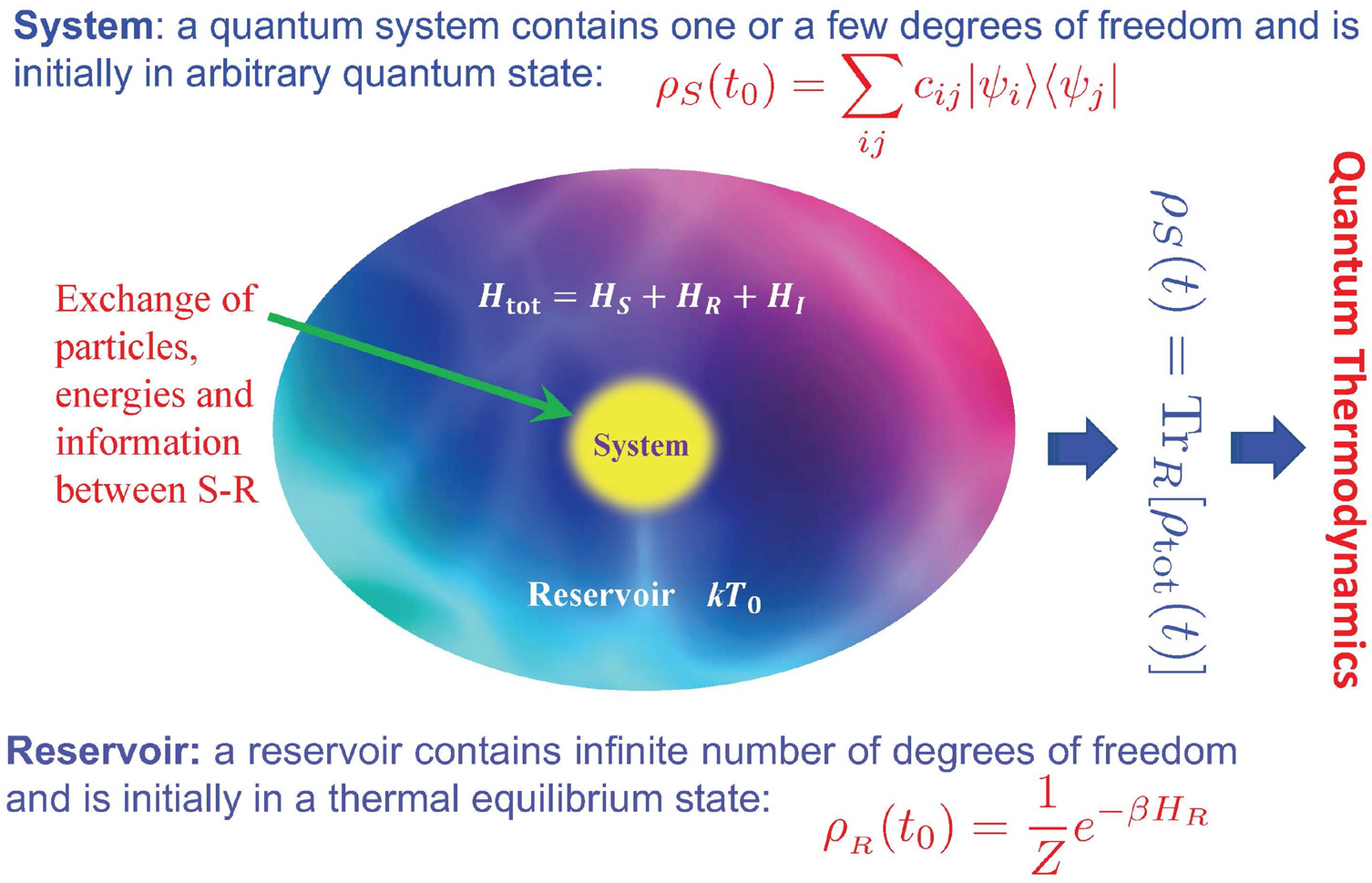

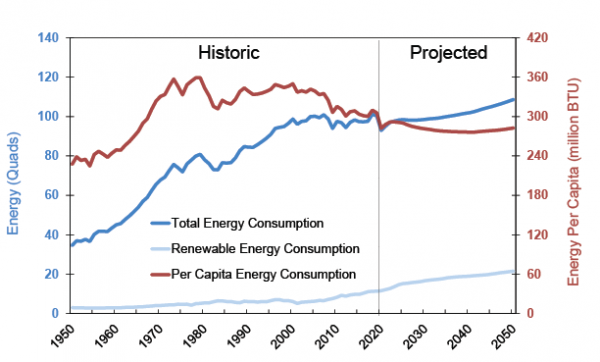







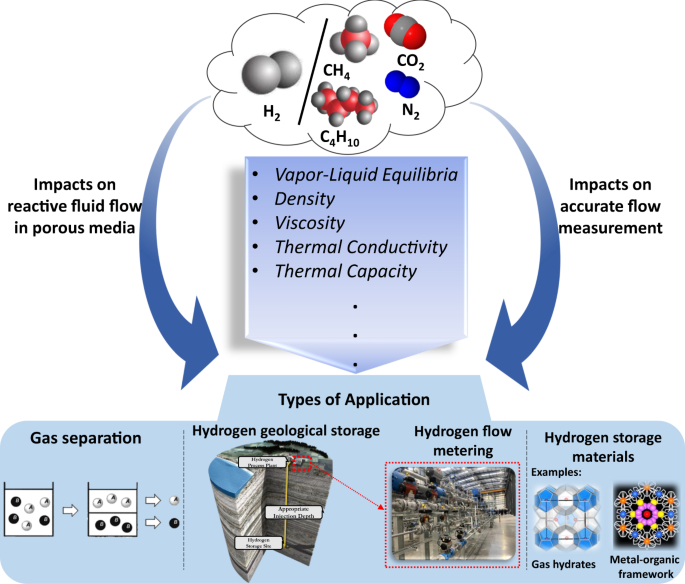











Post a Comment for "Can An Energy System Contain A Certain Amount Of Heat"